Biology concepts – protein moonlighting, undulipodia, evolution, basal body, centriole, GAPDH, intraflagellar transport
This phenomenon is called protein moonlighting. The re-evaluation of the human genome (about 19,000 genes) suggests that many proteins have more than one distinct function. This would allow for a relatively small number of genes to provide a large functional proteome (the total number of protein functions). As such, a 2014 study is showing the importance of moonlighting proteins in health and biology.
There are rules for a protein to have a legitimate second job. It’s only moonlighting if the two functions are unrelated, the functions can't be carried out by two different domains of the protein either. This would suggest a gene fusion event. The two functions must be independent, so ablating one doesn’t affect the other.
There are hundreds of examples of moonlighting proteins in the literature now, and more are sure to follow. There are examples aplenty within the glycolysis pathway; you know, the breakdown of sugar for energy. No fewer than seven of the ten glycolytic enzymes are known to have other jobs.
But as amazing as GAPDH is, today’s example of a multitasker is even more rare, in that the moonlighter is a complete structure, made of many proteins, and has two distinctly different jobs. What’s more, each function has its own exceptions. What's our structure of interest? The basal body, or perhaps I should call it the centriole.
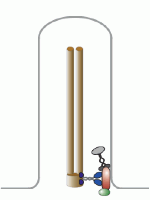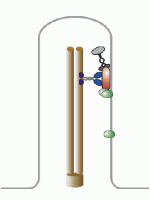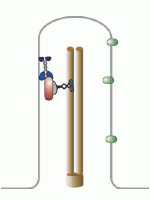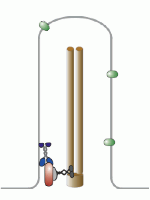
Let’s talk about the basal body first. This is the base of the eukaryotic undulipodia (cilia and flagella). These moving tails come in two parts; the basal body and the axoneme. We talked at length about the axoneme a few months ago, with its nine doublet microtubules surrounding two singlet microtubules (9[2]+2, see picture below). Undulipodia movement, as opposed to the motor driven prokaryotic flagella, is achieved by sliding the different doublets forward and back past one another.
But in those previous posts we didn’t talk much about the basal body. It too is a ring of microtubules, although these are shorter polymers that in the axoneme. Instead of doublets, there are almost always nine sets of triplet microtubules, and there are no center microtubules (9[3]+0). Of course, there were a couple of exceptions, and we talked about them.
we talked about last week, then it has hundreds of basal bodies as well – one per cilium.
The basal body serves as the nucleation site for mictrotubule growth into the axoneme. It’s like a skyscraper, the microtubule girders are built vertically on the basal body foundation; only here, the basal body sparks a self-assembly of the microtubules. You don’t need fearless guys climbing the beams to build an axoneme.
If we turn our attention to the centriole, we find that it’s used in mitosis. When a cell divides, each progeny cell receives one centrosome. Don’t confuse the centrosome with a centriole or a centromere (a near center point of a chromosome, it holds the two chromatids together). The centrosome is more of an area, it contains two centrioles, a mother and a daughter, and the pericentriolar matrix (PCM) amorphous group of proteins that help the centrioles do their job.
Each centriole is a complex microtubule formation in a 9(3)+0 arrangement, about 120 nm wide and 175 nm long. This is exactly the structure of the basal body – they’re the same thing! Well, almost.... a centriole has to mature into a basal body.
During S phase of the cell cycle (when the chromosome are replicated) the centrosome will duplicate. Each centriole grows another one from its side, at a right angle. The mother is a mother again, and the daughter becomes a mother for the first time. The two mother/daughter pairs then gather their own PCMs and move to the sides of the nucleus. When mitosis time comes, microtubules grow toward the chromsosomes, but from the mother centriole only. This is the spindle and will help pull the chromatids apart during cell division.
A commentary published in 2010 talks about how many cell types, including some oocytes can undergo meiosis without centrioles, while centriole numbers than are re-established after fertilization. However, in other tissues, loss of centrioles leads to genetic instability over time, even if the spindle will develop without the centrosome. To this point, we still haven’t resolved the issue of whether centrioles are necessary for proper cell division.
In the vast majority of cases, centrioles come from centrioles. One serves as a template to form the second. The process through which this occurs has just begun to be revealed in the past few years. There is a linking fiber from the proximal end of the mother centriole that acts as a seed point to start aggregation of the daughter centriole at a 90˚ angle to the first.
The process is very complicated, but is carried out spontaneously, without specific gene products to guide it. It was first believed that if centrioles were lost, then they could not be regained. Of course, they also thought that centrioles had their own DNA. Now we know that in cases where centrioles are lost, they can form de novo, and function just fine in mitosis or as basal bodies. In several studies, removal of centrioles or cells without centrioles to begin with allows for new centrioles being formed from aggregated microtubules.
A 2011 review shows that there are many proteins involved in the process. First the distal end of the centriole is capped, then it migrates to the cell membrane and docks. A transition zone is produced that allows for selective movement of molecules up and down the inside of the axoneme (intraflagellar transport, IFT).
Finally, there is attachment of accessory bodies like rootlets to anchor it to the membrane, transition fibers to move to the axoneme and distal appendages. Only after all this maturation of the basal body can the axoneme be built by IFT. This is how the process happens in all species EXCEPT fruit fly male gametes and the microbe Plasmodium yoelii, where the axoneme grows BEFORE plasma membrane docking of the basal body.
What’s more, you can always reverse it and go from basal body back to centriole, so their functions must somewhat overlap. The basal body and centriole both serve as microtubule organizing centers (MTOCs). So what makes it a moonlighting structure?
That’s a little weird, but what’s really weird is the kids. In a 1991 paper, part of the oviduct of a quail was reversed, so that the cilia beat toward the ovary instead of toward the uterus. When cells divided through embryo and chick development, the cilia of the progeny cells had the same orientation as those in the parent! They continued to beat in the wrong direction. Since basal bodies are duplicated from basal bodies, the mother basal bodies gave rise to daughters that beat the same direction.
The evidence presented shows that we have an ancient, yet complex, structure that has a couple of important jobs. The question for evolutionary biologists is which came first, the basal body or the centriole? The last common eukaryotic ancestor (LECA), the cell from which all eukaryotic cells descend, had undulipodia, so the basal body is an extremely old structure. But all eukaryotic cells undergo mitosis or meiosis, so the centriole must be important too.
 |
Notice the cilia that are the upper most in the animation. They show best the coordinated beating that pushes fluid in one direction. |
Cells without either centrioles and basal bodies suggest that basal body function came first; since you can’t have undulipodia without basal bodies, but our discussion above shows you can have mitosis without centrioles.
On the other hand, centrioles have to mature to become basal bodies, wouldn’t this suggest that they came first? Or perhaps the basal body was the primary product and nature managed to find a use for one if its precursors. What do you think, is the basal body the chicken or the egg?
Next week let’s look at one of the animal kingdoms great exceptions in terms of cilia, even though primitive and higher animals have them, round worms don’t cilia or flagella!
For more information or classroom activities, see:
Protein moonlighting –
Centriole –
Basal body –
Centrosome –