Biology Concepts – platyhelminthes, asymmetry, bilateral symmetry, evolution, cephalization, natural selection, fish, lepidophagy
Takeuchi, Y., Hori, M., & Oda, Y. (2012). Lateralized Kinematics of Predation Behavior in a Lake Tanganyika Scale-Eating Cichlid Fish PLoS ONE, 7 (1) DOI: 10.1371/journal.pone.0029272
Lee, H., Kusche, H., & Meyer, A. (2012). Handed Foraging Behavior in Scale-Eating Cichlid Fish: Its Potential Role in Shaping Morphological Asymmetry PLoS ONE, 7 (9) DOI: 10.1371/journal.pone.0044670
Kusche, H., Lee, H., & Meyer, A. (2012). Mouth asymmetry in the textbook example of scale-eating cichlid fish is not a discrete dimorphism after all Proceedings of the Royal Society B: Biological Sciences, 279 (1748), 4715-4723 DOI: 10.1098/rspb.2012.2082
Takahashi, T., & Hori, M. (2008). Evidence of disassortative mating in a Tanganyikan cichlid fish and its role in the maintenance of intrapopulation dimorphism Biology Letters, 4 (5), 497-499 DOI: 10.1098/rsbl.2008.0244
Hori, M., Ochi, H., & Kohda, M. (2007). Inheritance Pattern of Lateral Dimorphism in Two Cichlids (a Scale Eater, Perissodus microlepis, and an Herbivore, Neolamprologus moorii) in Lake Tanganyika Zoological Science, 24 (5), 486-492 DOI: 10.2108/zsj.24.486
What is the largest living structure on Earth? No, it’s not the 2200 acre Armillaria ostoyae fungus in Oregon that we talked about previously. That is the largest single organism, but there is something bigger.
This can add up quickly, because the Great Barrier Reef is more than 132,973 sq. miles (344,400 sq. km) in area. And if you still don’t believe it is a living structure, get this; it’s moving south! Climate change is warming the waters off the coast, and corals and tropical fish are moving south with the warmer temperature. Meanwhile, the northern edge recedes as the waters get too hot for corals.
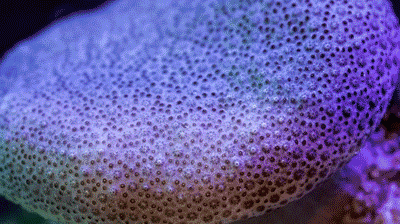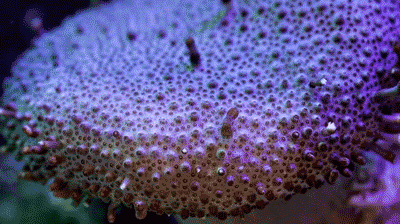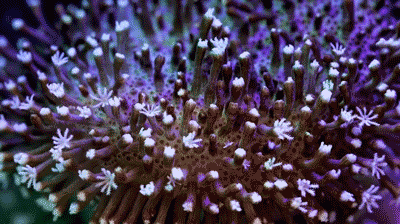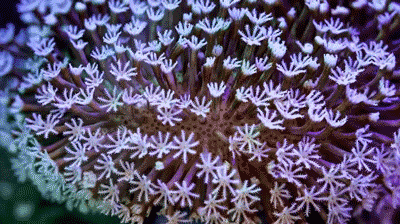
Corals take all sorts of shapes (see picture above), and despite what we talked about last week, they don’t seem to be either bilaterally or radial symmetric. Sure, brain corals look radial, but most corals don’t have a repetitive shape. Are these asymmetric animals?
Nope - remember that the coral you see is the exoskeleton of the polyp, not the animal itself. Just because the apartment building isn’t symmetric, it doesn’t mean the animal is as well. Coral polyps are definitely radially symmetric, so our discussion of last week still holds.
Corals and other radial animals are exceptions, since 99% of animals are actually bilaterally symmetric. But there are exceptions with the bilateral animals as well. Some species that have been bilateral for millions of years then evolved a tweak to the system. Some part them became asymmetric in order to give them an advantage. Their stories are exceptional and we should explore some of them.
Let’s start with the first animals that became bilaterally symmetric– the platyhelminthes, or flatworms. Most flatworms are small, just barely visible with the human eye, and most swim in the water. There are free-living versions and parasitic species and it is in the parasites that we find our first animal that has decided that completely bilateral isn’t necessary.
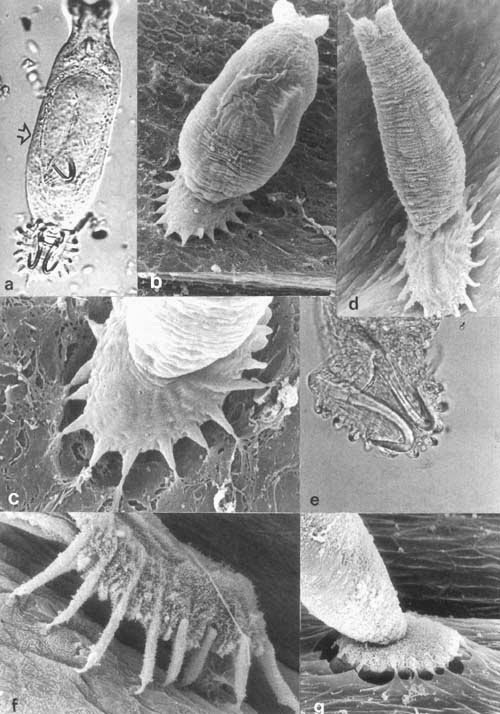
Polyopsithocotylea monogenea is a group of flatworms that live on and feed on fish gills. About 0.05-1 mm long, these platyhelminthes attach themselves to one of the gill ridges and take up residence there for life. The attachment they use is called a haptor, and they come in different shapes and sizes.
The oncomiracidium stage of the worm is directly after the egg stage and before it becomes an adult. This stage is completely bilaterally symmetric. But when the adult stage is reached and it’s time to settle down and starting feeding on some fish’s gills, they become asymmetric via their haptor attachment.
The initial haptor is usually located on the posterior end, on one side only. So much for bilateral symmetry. In some species this haptor has suckers, in others it has hard clamps, and yet other species have both.
As the adult grows, more haptors may develop, just where the animal touches the gill. Some may have 50 or more haptors arranged around their posterior, becoming more and more asymmetric. But there is a plan, they only grow where attachment is possible; some signal is generated by contact and this stimulates growth of more attachments.
Here we have a family of parasitic worms that aren’t symmetric living within a phylum of worms that were the first to be bilaterally symmetric - exceptional. But, take one step up the chain and you see the fish they live on. It just so happens that at least one group of fish parasitized by P. monogean worms are asymmetric themselves.
The cichlids are successful because different species have developed different feeding niches, and this is where we meet our asymmetric cichlids – they eat the scales off other fish! One genus, Perissodus, has at least six species that eat scales, all endemic to Lake Tangayiki (although there are also other scale eaters in other locations).
Scale ripping and eating is called lepidophagy (lepido = scale, and phagy = eat). Scales are an unexpectedly good source of nutrition. They chock full of protein and calcium phosphate, and their attachments are both cartilaginous, fatty, and come with some carbohydrate. Remember that the next time you order fish in a fancy restaurant. Tell the chef not to scale it – you’ll be quite the topic of conversation in the kitchen.
But, you can imagine that the fish being unfrocked don’t appreciate it very much and fight back or swim away quickly. That doesn’t even take into account how hard it is to bite the scales off a swimming fish. Therefore, lepidophages must evolve anatomies and behaviors that give them a chance to succeed. Or perhaps it would be better to say, they acquired characteristics that made being a lepidophage an advantage.
A 2012 video study showed that right-mouthed individuals almost always attack prey from the left, and their strikes are more powerful and successful when coming from the preferred side. One could ask, why are their both types? How did right- and left-mouthed individuals come to evolve and why are there still both types?
A different 2012 study shows that juveniles prefer one side or the other, even before their mouth bend has become pronounced, so it is a deeply penetrating characteristic, both heritable and perhaps partially acquired. There’s evolution and genetics at work here.
The prevailing model is that at any one time, right or left-mouthed individuals will predominate in a population. Let’s say that right now, right-mouthed feeders are the majority. The prey fish will learn to pay more attention to their left side, as this side is more vulnerable.
This makes it harder for right-mouthed individuals to feed, but easier for left-mouthed fish, because the prey fish ignore their right side relative to the left. In time, the right-mouthed individuals breed less well and the numbers in the population will shift. The left-mouthed feeders will become the majority. This is an example of negative frequency-dependent selection, where as a trait becomes more common it becomes less advantageous, and there is a balancing selection.
However, a 2012 study throws this into question. They found equal distributions of right-and left mouthed individuals in five populations they studied. Also, left-mouthed and right-mouthed individuals mated with each other just as often as they mate with same-sided individuals (called disassortative mating). Perhaps the mouth bend is not a true dimorphism (di= two, and morph = shape).
Or, as a 2008 study suggests, negative frequency-dependent selection works best when there is disassortative mating. This may be necessary since a 2007 study showed that lefty:lefty matings give 2:1 lefty offspring, right:lefty mates give equal righty and lefty offspring, but righty:righty pairs ONLY give righty kids. Figure out the genetics of that. The authors proposed two possibilities – mendelian genetics with lefty being dominant and dominant homozygous being lethal, orcross-incompatibility that is predominant in lefty:lefty homozygotes, (meaning lefty homozygotes can’t mate successfully mate with the other types).
Today we have seen a swimming flatworm that feeds on some fish, and some fish that feed on the scales of other fish. And both of them achieve this only because they have adapted their bilateral symmetry to become just a bit asymmetric.
Next week, there are other animals that break symmetry to survive. Flatfish lay on their sides at the bottom of lakes and oceans, yet they still use binocular vision. How can that be?
For more information or classroom activities, see:
Great Barrier Reef –
Coral polyps –
Cichlids –
http://www.cichlid-forum.com/articles/lake_tanganyika_diverse.phphttp://www.news.leiden.edu/news/super-fast-evolution.html