Biology concepts – photosynthesis, chlorophyll, pigmentation, astrobiology, exoplanet, dormancy
O'Malley-James, J., Raven, J., Cockell, C., & Greaves, J. (2012). Life and Light: Exotic Photosynthesis in Binary and Multiple-Star Systems Astrobiology, 12 (2), 115-124 DOI: 10.1089/ast.2011.0678
Kiang, N., Segura, A., Tinetti, G., Govindjee, ., Blankenship, R., Cohen, M., Siefert, J., Crisp, D., & Meadows, V. (2007). Spectral Signatures of Photosynthesis. II. Coevolution with Other Stars And The Atmosphere on Extrasolar Worlds Astrobiology, 7 (1), 252-274 DOI: 10.1089/ast.2006.0108
There is a large King Crimson Norway Maple (Acer platanoides 'King Crimson’) in our front yard. Healthy and round, it is a fine showpiece. We are also blessed with a 15-foot tall burning bush (Euonymus alata 'Compacta') not more than thirty feet from the maple. The burning bush straddles the property line with our neighbor, so when it needs work, its theirs, and when it is beautiful in autumn, it’s ours. Together, they make our landscaping come alive with color and provide ample shade.
They’re autotrophs (auto = self, and troph = feed) as are most plants. They make their own carbohydrates from sunlight, carbon dioxide, and water. Our sun radiates light energy that can be captured and transduced to chemical energy, but not all stars are the same and not all plants are green, so…..
Question of the Day: How can starlight support non-green plants and could it might it be different elsewhere?
Chlorophyll is one of several plant pigments, and chlorophyll itself comes in several flavors, but the primary plant chlorophylls are a and b. The “a” version is the major pigment for photosynthesis, absorbing light at the two ends of the visible spectrum – blues and reds (see picture). Green and yellow light get reflected, and this is what we see. Chlorophyll probably evolved to use red and blue because blue is high energy and red is abundant.
Chlorophyll b is an accessory pigment that plants use in smaller amounts. The “b” version absorbs light from near the same wavelengths as chlorophyll a, but they pass the energy on to the “a” version for use in photosynthesis. The two chlorophylls differ at only one of their 55 carbon atoms.
There are also chlorophylls c, d, and f. Chlorophyll c is also an accessory pigment which transfers energy to chlorophyll a, but it is very different structurally. Chlorophyll c is found only in some marine algae, and actually comes in three similar structures; c1, c2, and c3.
Chlorophyll f absorbs in the near infrared (NIR, not visible but close to red) range. Discovered in 2010 as the major chlorophyll in stromatolites of Australia, it is the first new chlorophyll identified in the last 60 years. However, its usefulness in photosynthesis has not yet been confirmed.
Chlorophyll d, on the other hand, is found to be the primary chlorophyll in cyanobacteria. A recent study showed that this chlorophyll absorbs NIR light as well. Though lower energy than red light, but the 2012 papershows that the cyanobacteria are just as efficient at photosynthesis as plants with chlorophyll a. This works out well since in water, the higher energy wavelengths are absorbed near the surface and the only light that penetrates to the cyanobacteria is the NIR.
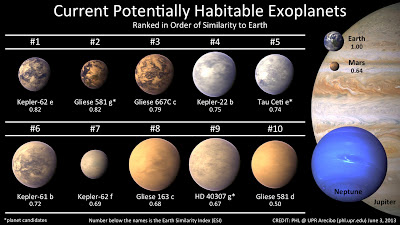
This is important for the science of astrobiology, predicting what life might look like on other planets and trying to identify which planets might hold life. Knowing that low energy light can still power photosynthesis tells us that we should not discount the planets around red dwarf stars. These stars have light of different wavelengths than our sun. Autotrophs from planets around red dwarfs may use NIR chlorophylls exclusively; therefore they might reflect all light and appear almost white.
On the other hand, light from different stars might drive evolution of different chlorophylls, so plants on other planets might not be green at all, but could reflect just lower energy light and appear red, or reflect just higher energy waves and be blue – blue plants, cool!
Based on the light reflected from exoplanets (planets outside our solar system), a 2007 study in the journal, Astrobiology, says we might be able to predict the color of their possible plants and the wavelengths they might use. Furthermore, a study in 2012 stated that in binary systems that have two stars, each giving off different wavelengths of light, might force the evolution of dual photosynthetic mechanisms, leading to perhaps alternating plant colors, depending on which sun is shining.
Chlorophylls provide energy through photosynthesis, but they also have a cost. The old saying, “It takes money to make money” applies to plants as well. It takes energy to make chlorophyll, so it only pays to make chlorophyll when there is ample sunlight to put through photosynthesis. When the daylight get shorter on Earth, the profit margin for producing chlorophyll goes down, so the plant just stops making it.
This is when we start to see the other pigments, those that might play a role on other planets. Other major pigments are the yellow, orange or red carotenoids and the flavonoids. When the plant reduces chlorophyll production, the green color is then a lower percentage of the total pigment in the leaf and the other colors can show through. This gives the bright colors of fall foliage.
But these same pigments can make it seem that a green plant is a non-green plant. Plants that produce large amounts of purple, brown, or maroon pigments have leaves that are so dark that they appear black. Purple, black, and red plants have chlorophyll aplenty, it’s just that the color is masked by other pigments.
Carotenoids are a diverse group of pigments, but yellows and oranges seem to predominate. Carrots get their color from carotene, one type of carotenoid. Xanthophyll is another, which reflects yellow light wavelengths. While chlorophylls absorb red and blue light, carotenoids absorb the blue wavelengths, as well as green light, reflecting only the lower energy yellow, orange, and red light.
By absorbing the green light that would usually be bounced back from chlorophyll, they can prevent us from seeing them as green. Additionally, non-green plant pigments can contribute to photosynthesis, serving as accessory pigments to chlorophyll.
Carotenoids absorb light energy, and while they can’t convert this directly to chemical energy through photosynthesis on Earth, they can transfer this energy to chlorophyll, which then carries it through photosytems I and II of photosynthesis.
In addition, some archaea use retinal (another pigment) to extract energy from the green wavelengths of light. So, why aren’t plants truly black? Wouldn’t it be most efficient to absorb all wavelengths of light for photosynthesis and reflect nothing, thereby appear black to us. Wouldn’t this be the most efficient use of the sun’s energy?
The answer is easy – evolution doesn’t work to maximum efficiency. Natural selection is random and works with what it is given – nothing in nature is engineered by decisionto maximize efficiency. But that doesn’t mean there can’t be black plants around other stars, having undergone completely different evolutionary paths.
Even if something used carotenoids, retinal, xanthins and chlorophylls, could it extract energy by absorbing all light waves that strike the plant? Um, no. No plant comes close to absorbing all the light that it can use, and no plant is made of only pigment molecules. There will always be reflections from other molecules.
Plus, if all light was absorbed, can you imagine how hot the plant would get? Imagine a blacktop parking lot being alive; you can fry an egg on an asphalt surface during the summer!
Carotenoids are longer lived than chlorophyll. When autumn comes around, the plant breaks down chlorophyll so that the components can be reused, but the carotenoids stick around much longer. Therefore, the yellows and oranges are not masked by the greens, and the leaves change colors.
Anthocyanins of the flavonoid class are another set of plant pigments. These colors are also more stable than chlorophylls. Our King Crimson Maple makes a lot of red anthocyanin pigments that absorb the green light coming in to the leaf and perhaps a lot of the green light reflected by the chlorophyll. Therefore, as the amount of the anthocyanins in a leaf increases, the green color is masked by the red.
Plants can use anthocyanins as “sunscreen” because in addition to absorbing green light, they also absorb ultraviolet light. Even though plants and animals need oxygen, they can also be damaged by the production of oxygen radicals (highly reactive compounds) produced by ultraviolet light energy striking oxygen-containing molecules and breaking them apart. Ultraviolet light can especially damage DNA, so anthocyanins can protect cells from mutations that might lead to inefficient activity or even cancer. It might be that on other planets, anthocyanins could be photosynthetic and plants live on UV light.
Sunscreen protects our skin from damage, just as red pigments protect the plant leaves. Even more, eating plants high in anthocyanins, like red grapes, blackberries, and blueberries, can transfer those antioxidant molecules to us for protection of our tissues and blood…. but don’t eat your Norway Maple.
When fall comes, or it is time for the fruits to ripen, plants start to produce even more anthocyanins (as in green apples turning red), because as other compounds in the plant breakdown more oxygen radicals will be produced. Therefore, the plant needs more protection.
Returning to our maple and our fire bush, it would seem that the maple leaves are dark red, almost purple, because of the high anthocyanin pigment concentration relative to the chlorophyll concentration (red + green = almost purple). But not all of them are purple (see picture). Other examples of this, the purple heart plant and the oxalis regnelli, remain purple all through their growing cycle.
Our burning bush is deep red in autumn because it is not shaded at all, so it produces more anthocyanin to protect its leaves in the summer. If it were shaded part of the time, it might be more pink. If the leaves need protection, they make more anthocyanin, and if not, they don’t. Don't ask me about shade on other planets.
Next week, your Fourth of July ice cream may have a side effect - ever wonder how "brain freeze" works?
Behrendt, L., Schrameyer, V., Qvortrup, K., Lundin, L., Sorensen, S., Larkum, A., & Kuhl, M. (2012). Biofilm Growth and Near-Infrared Radiation-Driven Photosynthesis of the Chlorophyll d-Containing Cyanobacterium Acaryochloris marina Applied and Environmental Microbiology, 78 (11), 3896-3904 DOI: 10.1128/AEM.00397-12
Next week, your Fourth of July ice cream may have a side effect - ever wonder how "brain freeze" works?
Behrendt, L., Schrameyer, V., Qvortrup, K., Lundin, L., Sorensen, S., Larkum, A., & Kuhl, M. (2012). Biofilm Growth and Near-Infrared Radiation-Driven Photosynthesis of the Chlorophyll d-Containing Cyanobacterium Acaryochloris marina Applied and Environmental Microbiology, 78 (11), 3896-3904 DOI: 10.1128/AEM.00397-12
Kiang, N., Segura, A., Tinetti, G., Govindjee, ., Blankenship, R., Cohen, M., Siefert, J., Crisp, D., & Meadows, V. (2007). Spectral Signatures of Photosynthesis. II. Coevolution with Other Stars And The Atmosphere on Extrasolar Worlds Astrobiology, 7 (1), 252-274 DOI: 10.1089/ast.2006.0108