Biology concepts - chirality, homochiral, enantiomer, stereoisomer, racemic mixture, biofilm, antimicrobial peptide, protein, amino acid
For every type of every subatomic particle there is an opposite particle. There are protons and anti-protons, electrons and anti-electrons (positrons), neutrons and anti-neutrons. Together, they make up matter and antimatter– think Star Trek.
Lam H, Oh DC, Cava F, Takacs CN, Clardy J, de Pedro MA, Waldor MK. (2009). D-amino Acids Govern Stationary Phase Cell Wall Re-Modeling in Bacteria Science, 18 (325), 1552-1555 DOI: 10.1126/science.1178123
Kolodkin-Gal I, Romero D, Cao S, Clardy J, Kolter R, Losick R. (2010). D-Amino Acids Trigger Biofilm Disassembly Science, 328 (5978), 627-629 DOI: 10.1126/science.1188628
Sasabe J, Miyoshi Y, Suzuki M, Mita M, Konno R, Matsuoka M, Hamase K, Aiso S. (2012).
D-amino acid oxidase controls motoneuron degeneration through D-serine. Proc Natl Acad Sci U S A. , 109 (2), 627-32 DOI: 10.1073/pnas.1114639109
Simanski M, Gläser R, Köten B, Meyer-Hoffert U, Wanner S, Weidenmaier C, Peschel A, & Harder J (2013). Staphylococcus aureus subverts cutaneous defense by d-alanylation of teichoic acids. Experimental dermatology, 22 (4), 294-6 PMID: 23528217
Errico F, Napolitano F, Squillace M, Vitucci D, Blasi G, de Bartolomeis A, Bertolino A, D'Aniello A, & Usiello A (2013). Decreased levels of d-aspartate and NMDA in the prefrontal cortex and striatum of patients with schizophrenia. Journal of psychiatric research PMID: 23835041
For every type of every subatomic particle there is an opposite particle. There are protons and anti-protons, electrons and anti-electrons (positrons), neutrons and anti-neutrons. Together, they make up matter and antimatter– think Star Trek.
So why is our universe made of matter and not antimatter? It turns out that when matter meets antimatter, they obliterate one another. This is bad – Scotty was always trying to prevent a matter/antimatter catastrophe on the Enterprise.
When the universe was young, there was antimatter and matter; lots of annihilations. Scientists hypothesize that there were a few more molecules of matter than antimatter, so when the fireworks were over, only matter remained; so matter matters.
What’s this got to do with protein exceptions, our subject for last week and this? We’ll get to that, a little background first. Remember that the shape of the protein is important for its function, and its shape is dependent on the amino acid order and the structure of those amino acids.
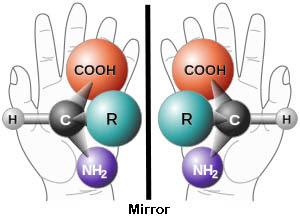
Look at your hands. They’re mirror images of one another, but no matter how you try, you can’t make your left glove fit exactly on your right hand. Unlike a ball reflected in a mirror where the two images will be superimposable on one another, there is no way to do this with say….. your left and right shoes.
The different enantiomers (enantio = opposite) have different chemical properties. Without getting into too much chemistry (nobody wants that), different enantiomers cause light waves to rotate in different directions.
One version of a molecule called gluteraldehyde rotates light to the right (dextrorotary or D, dextra = right), while the other is levorotary (levo = left). Amino acid enantiomers are comparable to the structure of gluteraldehyde, so amino acids that parallel the two gluteraldehydes are assigned a D- or L- label, ie. L-alanine has a structure similar to the gluteraldehyde enantiomer that rotates light to the left.
This is a bit of a misnomer because light rotation depends on many factors. In fact, many amino acids labeled L-type because of their similarity to L-gluteraldehyde actually rotate light to the right –that little factoid won’t be on anyone’s final exam.
Most proteins fold on their own, and they fold the same way every time. But what might happen if the protein sometimes used a certain L-amino acid and sometimes the D-version? The two resulting proteins would fold differently and therefore have different possible functions. Heaven forbid!
To avoid this, nature has devised an ingenious solution - only L-amino acids are used. This assures that all proteins of a specific type assume the same shape, and it turns out that proteins are most stable when they are made of all L- or all D-amino acids. This is the rule of homochirality.
One question before the exceptions -just why did life opt for all L-proteins instead of all D-proteins? Do they annihilate one another when they come into contact, like matter and anti-matter? Thankfully, no.
Were there more L-amino acids available on the early Earth so life made a choice and stuck with it? Maybe – this is one of the hypotheses currently being investigated. It’s also possible that some life developed as D-protein makers, but they were out-competed somehow and we descended from the L-protein winners. It isn’t as easy a question as the physics matter/antimatter issue - more on this biology exception next week. There are so many more exceptions in biology as compared to physics – that’s why I love life more than physics itself.
The most mundane is exception to homochirality is the one amino acid that isn’t chiral. Glycine’s R group is just a hydrogen, so the central carbon has two bound hydrogens, and the mirror images can be superimposed. There’s no L-glycine or D-glycine, just glycine. Don’t worry, the rest of the exceptions are better.
It turns out that the rule of homochirality is more of a guideline - there are examples of important D-amino acids (D-aa) in plants, animals, and prokaryotes. We don’t have time or space to go into many of them, but I will highlight two exceptions that are simply amazing. Let’s start with the bacteria since they own the planet, and we probably inherited our D-amino acid uses from them.
Bacteria use D-amino acids in various ways. First, new research shows that as bacterial numbers go up and resources (food) go down, D-aa-containing proteins might prepare bacteria for the bad times ahead. A 2009 study shows that Bacillus subtilisand Vibro cholerae make large amounts of D-aa as they age, including D-tryptophan, D-tryosine, D-phenylalanine, and D-leucine. As the amounts increase, they start to have an effect on the bacterial cell wall.
What is more, a 2010 study showed that in B. subtilis and S. aureus, the increasing numbers of bacteria and their increasing concentration of D-aa’s leads to a breakdown of the extracellular matrix that holds all the bacteria together (the biofilm). D-aa's were able to prevent biofilm formation and degrade existing biofilm, again preparing the bacteria to go off on their own as resources dwindle.
S. aureus also uses D-aa-proteins to avoid being killed. We have antimicrobial peptides (AMP) on our skin and mucosal surface that are always looking to kill bacteria. They often work by poking holes in the bacterial membrane. However, a 2013 studyshows that by switching out L-alanine to D-alanine in its cell wall, S. aureus can render the AMP’s ineffective. The D-alanine gives the protein a different shape, so the AMP's can’t fit in and do their job. Smart bacteria.
Animals have gotten into the act, especially the gastropod mollusks, ie. some snails and sea hares (marine slugs). D-tryptophan is their exception of choice. The cone snails (genus Conus), the most predatory and venomous of the marine snails, use D-tryptophan in the active peptides of their venom, called contryphans. Large cone snails have been known to kill humans, but the role of the D-tryptophan in the venom activity is not yet known.
How about us? Do humans use D-amino acids? Sure we do. But there are tricksters here. D-alanine is found in all mammals, but we don’t know what it might be doing. Its levels change with the time of day (circadian cycling), so who know what it might be controlling. A 2013 study set out to determine what drove the circadian changes. They tested several things: changing diet or fasting didn’t matter; enzymes that degrade D-alanine didn’t change levels of function either. But when they studied germ-free mice, the D-alanine levels didn’t change.
The scientists determined that it’s our gut bacteria making D-alanine, and circadian changes in intestinal absorption rates is the reason that the D-alanine levels fluctuate. It doesn’t mean that D-alanine isn’t doing something, but it sure had us fooled for a while.
Mammals don't stop there; D-serine and D-aspartate are so important, we have special enzymes called racemases whose job it is to convert their L-forms to D-forms (racemic mixtures contain both D- and L-versions of a molecule). The most amazing exception must be D-serine. However, we will see that there is a Goldilocks effect to D-aa’s, you don’t want too much or too little.
A certain brain neuron receptor (called NMDAR, important for learning and memory) is activated by an amino acid called L-glutamate, but it needs help from either glycine or D-serine to set off the electrical impulse. It turns out that in Lou Gehrig’s disease (amyotrophic lateral sclerosis), lower motor neurons die because they undergo aberrant excitation. In genetic cases of ALS, patients have too much D-serine!
A 2012 study showed that D-amino acid oxidase (DAAO) is mutated in these patients and doesn’t do its job of breaking down D-serine. Mice with a mutated DAAO were shown to have decreased lower motor neurons and more ALS signs.
So too much D-Ser is bad, but how about too little? Many studies have shown that schizophrenia patients have low levels of D-Ser. It might be that DAAO is too active, or perhaps a D-Ser racemase is inactive, or maybe there is just too little L-Ser to make D-Ser from – we don’t know yet.
A 2013 study has also implicated D-aspartic acid in schizophrenia. D-Asp can replace L-glutamate in activating NMDA receptors, and schizophrenic patients have low D-Asp levels in the brain and blood. The D-Ser and D-Asp data implicate glutamergic receptor activation in schizophrenia, so much work is underway to find ways to increase these D-aa’s in our brains - the very things that the rules say we shouldn’t be using in the first place. But let’s not raise them too much, no one wants ALS!
Next week, we switch our attention to carbohydrates, the energy sources in our cells. Every cell on Earth is designed to make ATP from glucose - except for those cells that ONLY use fructose.
Sasabe J, Miyoshi Y, Suzuki M, Mita M, Konno R, Matsuoka M, Hamase K, Aiso S. (2012).
D-amino acid oxidase controls motoneuron degeneration through D-serine. Proc Natl Acad Sci U S A. , 109 (2), 627-32 DOI: 10.1073/pnas.1114639109
Simanski M, Gläser R, Köten B, Meyer-Hoffert U, Wanner S, Weidenmaier C, Peschel A, & Harder J (2013). Staphylococcus aureus subverts cutaneous defense by d-alanylation of teichoic acids. Experimental dermatology, 22 (4), 294-6 PMID: 23528217
Errico F, Napolitano F, Squillace M, Vitucci D, Blasi G, de Bartolomeis A, Bertolino A, D'Aniello A, & Usiello A (2013). Decreased levels of d-aspartate and NMDA in the prefrontal cortex and striatum of patients with schizophrenia. Journal of psychiatric research PMID: 23835041
For more information or classroom activities, see:
Chirality –
Bacterial cell wall –
Biofilm –
Antimicrobial peptides –
D-amino acids –
Amyotrophic lateral sclerosis –
http://www.mayoclinic.com/health/amyotrophic-lateral-sclerosis/DS00359/DSECTION=treatments-and-drugs
Schizophrenia -