Biology concepts – tardigrade, cryptobiosis, anhydrobiosis, eutely, hyperplasia, hypertrophy, dry vaccine, dormancy
Next week, just how do you describe the climate of your hometown? Biomes are scientific entities, but it seems we can't agree on what each looks like or is called.
Borde, A., Ekman, A., Holmgren, J., & Larsson, A. (2012). Effect of protein release rates from tablet formulations on the immune response after sublingual immunization European Journal of Pharmaceutical Sciences, 47 (4), 695-700 DOI: 10.1016/j.ejps.2012.08.014
McGee, M., Weber, D., Day, N., Vitelli, C., Crippen, D., Herndon, L., Hall, D., & Melov, S. (2011). Loss of intestinal nuclei and intestinal integrity in aging C. elegans Aging Cell, 10 (4), 699-710 DOI: 10.1111/j.1474-9726.2011.00713.x
Guidetti, R., Altiero, T., & Rebecchi, L. (2011). On dormancy strategies in tardigrades Journal of Insect Physiology, 57 (5), 567-576 DOI: 10.1016/j.jinsphys.2011.03.003
In the early 1980’s, a forerunner to the mixed martial arts craze was temporarily popular in the United States. Called the “ToughMan” contests, non-boxers would enter the ring for fights against other nonprofessional fighters. The rules were supposedly the same as in boxing, although many contests were run without proper supervision, and the tournaments sometimes required a participant to fight several times in one evening. Think legalized bar fights.
Famous participants in the toughman contests included “Butterbean” Esch – a 350 lb. boxer with one punch and an iron jaw, and of course, who can forget Mr. T. The contests are still held in many states, but MMA now has the majority of the fan base, even more than traditional boxing between trained fighters.
These guys were tough, but were they the toughest? Humans as a rule are weak for their size, scared of more things than they should be, and less inclined to fight to the death for a morsel of food or potential mate – well most are. So…..
Questions of the Day: What is the world’s toughest animal?
Ask a hundred people and you may get a hundred different answers. The bull elephant can fight off an entire pride of lions and can lift five tons. But can you really give the prize to an animals that is scared of a mouse?! Maybe they aren’t afraid of mice, but they will avoid them if possible, according to one of the more scientifically consistent episodes of Mythbusters.
A good second choice might be the honey badger. It supposedly knows no fear, and proves it by depriving lions of the prey they just killed. In one case, three honey badgers stole a entire carcass from seven lions! The South Africa National defense calls their armored personnel vehicles ratels, the afrikaans word for honey badger.
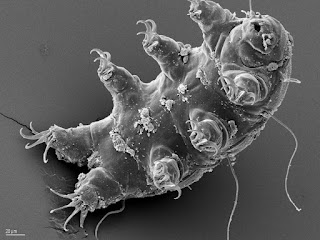
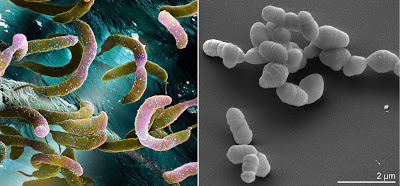
But I will try to convince you that a type of bear is the toughest animal on the face of the Earth, a water bear to be specific. This animal has long claws on each foot and a mouth that takes up a good portion of its head. On the other hand, it's less than 1 mm long!
The water bear is more scientifically known as a tardigrade (latin for slow walker), a phylum that falls somewhere near arthropods and nematode worms. There are two classes (eutardigradia and heterotardigradia) and more than 900 species, but there may be some overlap in those descriptions.
The adult tardigrade will have 40,000 cells, and will never have more. What is more, everyspecies of tardigrade is matures with a specific number. This is called eutely (eu = good, and telos = end). Many lower organisms may be eutelic; their cells have a limited number of divisions, so they grow to that number and then stop.
It isn’t just whole organisms that might be eutelic, organs can be as well. For example, the nematode AscarisALWAYS has 162 neurons. The research model nematode C. elegans has exactly 959 somatic cells, although a 2011 study has shown that C. elegans can lose critical cell nuclei as they age – tell me about it. Other nematodes, rotifers, and gastrotrichs have also been shown to have cell constancy at the body and/or organ level.
Tardigrades do grow after they reach adulthood, just not by adding cells. Growth by additional cells is called hyperplasia (excess formation), while growth by existing cells becoming larger is called hypertrophy (excess nourishment).
Prostate enlargement is often due to an increase in cells, hence the name benign prostatic hyperplasia, but hyperplastic growth doesn’t have to be pathologic. When you lose part of your liver, some can grow back by through hyperplasia. Likewise, hypertrophy is great when it is your muscles getting bigger, but not necessarily so good when your heart’s ventricles overgrow (ventricular hypertrophy).
Different tardigrade species are adapted to nearly every environment on Earth. They live in the Arctic and the Antarctic, in the mountains and the oceans, in the deserts and the jungles. All are found near water, some marine and some limnal(freshwater), some in the water and some just next to the water held in mosses or lichens.
But wherever you find them, you’ll find them in great numbers. The density of tardigrades can approach two million per square meter. Yellow crazy ants (Anoplolepis gracilipes) form supercolonies of incredible density, yet they can only muster about 2000 individuals per square meter. Haven’t heard of crazy ants? You will – look them up.
Tardigrade toughness doesn’t come from their pursuit of prey or their ability to fend off predators, but their willingness to live in conditions that would kill anything else, and I mean anything, else.
Cold, not a problem. Tardigrades can have liquid nitrogen (-346˚F/-210˚C) poured on them and they’re just fine. Heat – boil them for a couple of hours and then watch them lay eggs and go back to eating. Radiation isn’t a problem either; they can take 5700 grays of ionizing radiation without blinking.... well, they could if they had eyes. Humans curl up in a ball and die when exposed to 5 gray.
Some tardigrades live in black smokers at the bottom of the ocean, yet most of them can take 6000x normal pressure in stride. To sum it all up, in 2007 the Russians fired tardigrades into space for 12 days (near absolute zero, total vacuum, cosmic radiation). They came back and starting having babies. Now that’s tough.
How do they manage these amazing feats? Basically – they die and then come back to life. Technically, it’s called cryptobiosis (hidden life), but death and self-resurrection is not a bad description. During cryptobiosis, metabolism is reduced by 1000x fold or even more, down to the level where there is NO detectable chemical activity.
There are five recognized types of cryptobiosis, based on the noxious environmental condition that triggers it – anhydrobiosis (without water), chemobiosis(chemicals), cryobiosis (cold), anoxybiosis (lack of oxygen), and osmobiosis (change in osmotic potential).
The primary form for tardigrades is anhydrobiosis. They drop their claws, retract their legs and roll up into a ball called a tun. 99% loss of water, roll up into “tun” this is important because it regulates the rate of evaporative water loss. At this level, they don't hold enough water for damaging reactions to take place or even enough water to form ice crystals. The water is replaced by a sugar called trehalose.
Trehalose production is similar to the way many organisms can protect their structures and biochemistry from environmental damage, but apparently scientists have just touched the surface of how tardigrades react to uncomfortable environments. A 2013 study indicates that there are many unidentified organic molecules present in the tuns of tardigrades that are not present in the organisms under normal physiologic situations.
Amazingly, tardigrades are the only animal that can undergo all five types of cryptobiosis. It's really like dying and coming back to life. Reviving from cryptobiosis can take a little while, usually the longer they have been in anhydrosis, the longer it takes to recover. They can’t survive this way forever either.
Earlier reports had professed that 120 year old tardigrades were revived from dried lichen and moss samples in the British Museum, and that decades old samples were just fine. But Dr. James Garey of the University of South Florida tells me that many of these reports have been called into question and cannot be repeated.
Dr. Garey’s estimate is that tardigrades can survive 1-5 years as a tun, with decreasing viability upon hydration after that. Still, could any other animal you know of be dead for five years, with no air, no water, high radiation, liquid nitrogen, and taunts about their size and lineage – and then come right back to life when the opportunity is right?
Cryptobiosis is quite different than dormancy. Dormancy doesn’t bring a huge change in physiology – like 99% dessication. Also, dormancy is preemptive while cryptobiosis is reactive. However, a very good 2011 review of tardigrade reactions shows that they can undergo both dormancy and crytobiosis – sometime simultaneously!
The question is – how do they survive the bad conditions WHILE they are forming the tun? It takes about 20 minutes for tun formation to occur, so it appears that many of the conditions they can endure require them to already be in the cryptobiologic state. They can survive the radiation whenthey are dessicated, they can survive boiling when they are dessicated. I don’t think it makes them any less amazing.
Tardigrades aren’t even considered extremophiles, since they are not designed to live in extreme environments. But this is precisely why I think they are so tough, because few of them are adapted to extreme conditions, but they can survive deadly situations anyway.
Can the exploits of this microanimal help humanity? You betcha. Tardigrades' ability to undergo anhydrobiosis has begun to influence the design of medicines. In third world countries, a lack of reliable refrigeration requires vaccines and medicines don’t need refrigeration, and can be reactivated upon ingestion.
Dry vaccines are a current goal, so the National Institutes of Health recently put out a call for proposals for research into more thermostable and reactivateable preparations. A late 2012 paper has identified a tablet form for deliver of some protein drugs, with reactivation of the molecules with saliva. This would be much better than the current reliance on hypodermics and refrigeration. So tardigrades are tough for themselves, and may fight for us as well.
Borde, A., Ekman, A., Holmgren, J., & Larsson, A. (2012). Effect of protein release rates from tablet formulations on the immune response after sublingual immunization European Journal of Pharmaceutical Sciences, 47 (4), 695-700 DOI: 10.1016/j.ejps.2012.08.014
McGee, M., Weber, D., Day, N., Vitelli, C., Crippen, D., Herndon, L., Hall, D., & Melov, S. (2011). Loss of intestinal nuclei and intestinal integrity in aging C. elegans Aging Cell, 10 (4), 699-710 DOI: 10.1111/j.1474-9726.2011.00713.x
Guidetti, R., Altiero, T., & Rebecchi, L. (2011). On dormancy strategies in tardigrades Journal of Insect Physiology, 57 (5), 567-576 DOI: 10.1016/j.jinsphys.2011.03.003