Biology concepts – umami, taste, flavor, gustation, glutamate, chemoreception, CD36, fat taste, water receptor, calcium
Newman L, Haryono R, & Keast R (2013). Functionality of fatty acid chemoreception: a potential factor in the development of obesity? Nutrients, 5 (4), 1287-300 PMID: 23595136
Pepino MY, Love-Gregory L, Klein S, & Abumrad NA (2012). The fatty acid translocase gene CD36 and lingual lipase influence oral sensitivity to fat in obese subjects. Journal of lipid research, 53 (3), 561-6 PMID: 22210925
Keller KL, Liang LC, Sakimura J, May D, van Belle C, Breen C, Driggin E, Tepper BJ, Lanzano PC, Deng L, & Chung WK (2012). Common variants in the CD36 gene are associated with oral fat perception, fat preferences, and obesity in African Americans. Obesity (Silver Spring, Md.), 20 (5), 1066-73 PMID: 22240721
Chan KQ, Tong EM, Tan DH, & Koh AH (2013). What do love and jealousy taste like? Emotion (Washington, D.C.), 13 (6), 1142-9 PMID: 24040883
MacDonald CJ, Meck WH, & Simon SA (2012). Distinct neural ensembles in the rat gustatory cortex encode salt and water tastes. The Journal of physiology, 590 (Pt 13), 3169-84 PMID: 22570382
Until 1908, science believed that most flavors were just combinations of the four traditional tastes - sweet, salty, sour, and bitter. But don’t get the idea that taste and flavor are the same - oh no. Taste is our gustatory sense, but that isn’t the same as flavor– flavor is something bigger than taste.
You know how having a head cold makes food bland? Well, that’s because smell is a big part of flavor; you don’t smell your food when you have a cold. Food stimulates all your senses - temperature, touch, smell, what it looks like and even how it sounds as you chew it. All these things add up to flavor.
This is why chefs say you eat with your eyes first, and why they try to incorporate different textures into a single dish. They’re trying to appeal to all your senses. Therefore, eat slowly to enjoy your food more. Give all your senses time to participate. And you might just eat less, since your satisfaction will come from the total experience, not just the craving for a particular taste.
But back to the origins of umami. The Japanese had an idea that there was another taste, mostly since their traditional cuisine used so much seaweed, and this flavor couldn’t be accounted for by the other four tastes. Chemist Kikunae Ikeda wanted to identify the active molecule in seaweed, the one that gave it taste. He called it umami, from the Japanese words for delicious (umai), and taste (mi).
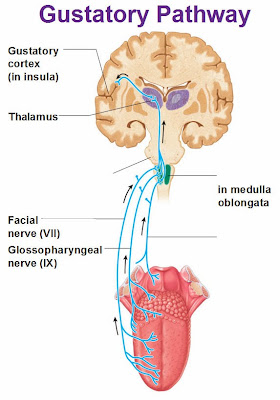
Ikeda’s biochemical studies led to the identification of glutamates as the molecules to which people reacted. And they aren’t just in seaweed, most living organisms contain truckloads of glutamates. When cooked, all glutamates convert to L-glutamate, the amino acid. Ikeda determined that this is what some of our gustatory (taste) receptors sense. Gustation is from Latin gustare = taste, and this is where the word gustocomes from; to taste life.
This is why identifying an umami taste receptor for L-glutamate makes sense. This is nature telling you that you need to eat protein, and by giving it a favorable neural response (it tastes good), it increases the chances that you will seek out protein sources for nutrition.
Glutamate has several functions, even beyond its role as one of twenty protein building blocks. Glutamate is the most common neurotransmitter in the central nervous system, and plays a crucial role in long-term potentiation (LTP) and learning. Glutamate is also an intermediate in synthesizing many of the molecules in glycolysis, gluconeogenesis, and the citric acid cycle. I’ve said it before and I hope it jumped into your mind just now – nature hates a unitasker.
So you sense L-glutamate through a gustatory receptor that is specific for that molecule, and the electrical impulse is converted to a specific taste – we call it savory. The cloning of the taste receptors in the late 1990’s (actually umami was the first) started people thinking about other possible tastes. Could there be a sixth taste sense – how about fats? Do we taste fats?
It has been shown that fatty acids in the oral cavity do have a threshold level for sensation, and that the fatty acid taste receptors do lead to specific changes in physiology. When subjects were given fatty acids on their tongues, they very quickly showed increased serum triglyceride levels, increased pancreatic hormone release, increased release of GI lipases (enzymes to breakdown fat) and a slowly of the GI tract (it takes more time to digest fats).
What is lacking here is a conscious perception of the discernable nature of the fatty acid (like how sugars are sweet or glutamates are savory). The 2009 studies by Mattes and colleagues controlled for the mouth feel, smell, and so forth of fats, so it was definitely the fatty acid receptor that was stimulating the responses, but no where did it say the subjects tastedsomething. However, his 2011 paper says that fat may very well be a basic taste.
This gives us a new way thinking about taste receptors. Taste is a type of chemoreception, but perhaps it’s only one subset of oral chemoreception. Gustatory chemoreception is a lot more than just tasting something. However, some researchers challenge this division, saying that participants do have a measurable psychophysical response when fatty acids on the tongue reach a threshold level – they dotaste something.
When divided into groups based on which variant of CD36 they possessed, a couple of studies from 2012 (here and here) show that responses to fat and how much the subjects craved fats were different. Those who sensed fats most readily (at lowest concentrations) tended to eat less than those who needed more fat in order to trigger the responses.
The hypothesis of a 2013 review of fat taste and obesity says that those who sense less fat are more likely be tipped toward a hunger stimulating hormonal profile, while those who more readily sense the fatty acids in their food tip toward satiety (fullness). There are many hormones involved here and we could get bogged down very fast, so let’s leave it at that for now; undoubtedly someone is trying to make a diet pill based on it.
So, could there be more oral chemoreception events going on – a seventh taste? An eighth? Let’s talk very briefly about two possibilities. Maybe we can taste calcium. Yes, you could taste your Tums. A 2008 study indicated that mice can perceive calcium as a specific taste. A single 2012 study extends this to humans as well. Calcium is sensed via a certain receptor (Tas1R3), which works with other proteins to sense sweet and umami. But here, it apparently works on its own (we will talk more about the receptors in the posts to come). I need to see more research before I buy calcium taste completely.
Taste number eight - do you think you can taste water? The common argument is that you can taste what is in the water, not the water itself. How or why would you taste water; you’re 65-70% water all the time! What good is it to taste the main ingredient of life? How about this – do you sense the water by taste receptor, not just by temperature, sound, smell, or mouth feel?
Taste number eight - do you think you can taste water? The common argument is that you can taste what is in the water, not the water itself. How or why would you taste water; you’re 65-70% water all the time! What good is it to taste the main ingredient of life? How about this – do you sense the water by taste receptor, not just by temperature, sound, smell, or mouth feel?
What is the purpose for water receptors in the oral cavity (really, they are in the entrance to the throat, the laryngeal pharynx)? It may be that this is an evolutionary protection from aspirating(breathing in) liquid to the lungs. Liquid in the lung is a bad idea, since it stops gas transfer and promotes bacterial growth. If acids or other liquids that could damage the lungs or throat get in their somehow, it would definitely be better to swallow them than to breathe them in.
When you were a fetus and a very young infant, stimulation of the water receptors in your throat caused you to swallow immediately. As you aged and gained more muscular control, the reflex was replaced by coughing – this is the hypothesis of a 2001 study on the reflex. But the water receptors are still there and still aid you as a stimulus for voluntary swallowing. Whether we taste water or not, I’m glad I have the chemoreceptors.
So, the dampening of the reflex by Cl- in salt might be helpful keeping you from constantly having the urge to swallow. This leads to another point – the power of suggestion. Can you do anything right now other than think about the saliva in your mouth and whether you should be swallowing? Creepy, isn’t it.
Don’t count out the idea of water as a basic tastant (something you can taste). A 2010 study showed by monitoring brain waves that people respond to water using the same pathways as taste, and the responses look the same. And a 2012 studyindicates that rats have distinct portions of the gustatory cortex of the brain for identifying both salt and water. If we can taste umami to make sure we eat enough protein, and sweet to make sure we eat enough carbohydrate, why not water to make sure we keep hydrated?
December 2013 experiment were asked to think or write about love, hate, or jealousy. Then they were asked to describe the taste of a new product (really just distilled water). Those who wrote or thought about love rated the water to be sweeter than those who contemplated hate or jealousy.
It seems that the brain pathways for rewarding feelings in love and in consuming sweet are the same. You can’t discern between the two, and one can stimulate the other. So when you say you love eating sweets, maybe you really do!
Next week, we can go further into taste. Do people who are supertasters taste good or taste well?
For more information or classroom activities, see:
Umami –
Fat taste –
Water receptor –