Biology concepts – TRPA1, cold sensing, oxygen sensing, proteasome function, hypoxia, normoxia, hyperoxia, phototransduction, optogenetics, methyl anthranilate
Bellono, N., & Oancea, E. (2013). UV light phototransduction depolarizes human melanocytes Channels, 7 (4) DOI: 10.4161/chan.25322
Bellono NW, Kammel LG, Zimmerman AL, & Oancea E (2013). UV light phototransduction activates transient receptor potential A1 ion channels in human melanocytes. Proceedings of the National Academy of Sciences of the United States of America, 110 (6), 2383-8 PMID: 23345429
Saito S, Banzawa N, Fukuta N, Saito CT, Takahashi K, Imagawa T, Ohta T, & Tominaga M (2014). Heat and noxious chemical sensor, chicken TRPA1, as a target of bird repellents and identification of its structural determinants by multispecies functional comparison. Molecular biology and evolution, 31 (3), 708-22 PMID: 24398321
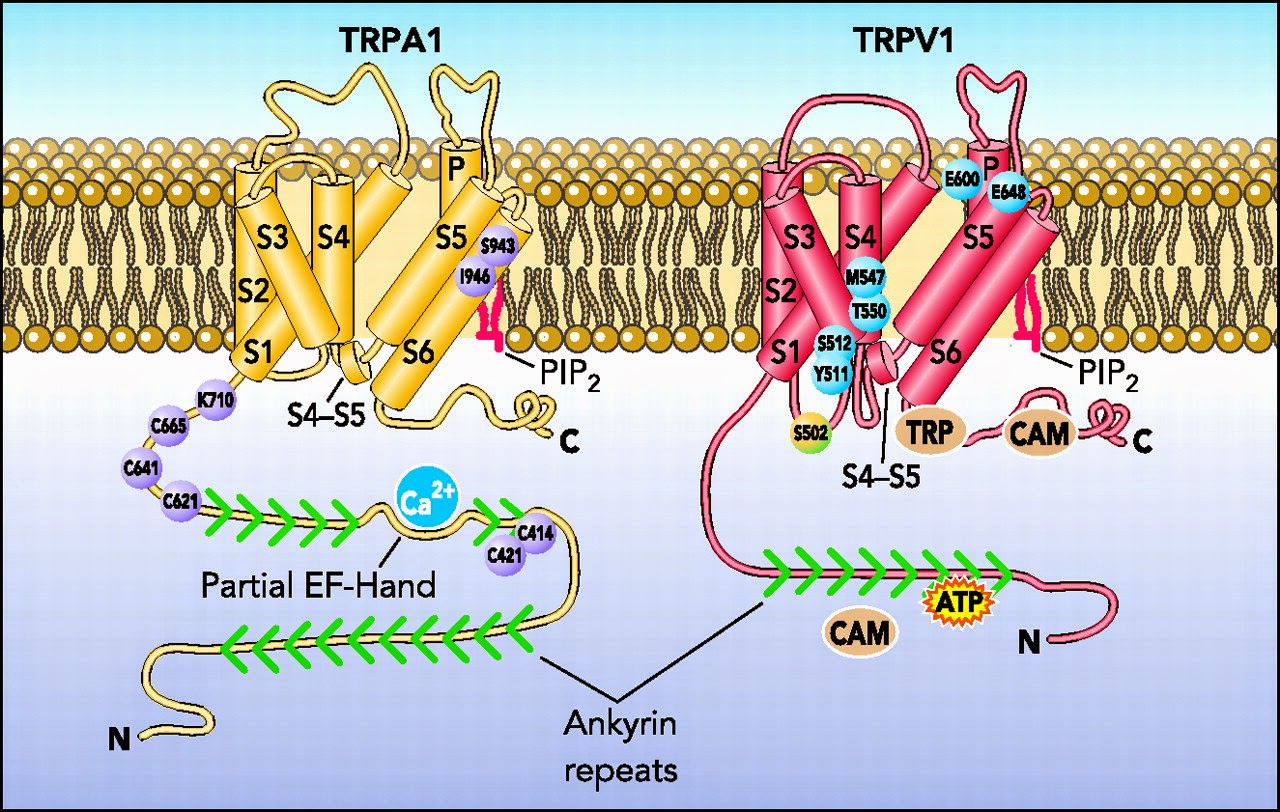
Last week we learned that the TRPA1 ion channel causes you pain when you are too cold and helps you to avoid the cell damage that cold can produce. Even if that’s all it did, it would be pretty amazing, but there’s much more. It turns out that TRPA1 can do some amazing things - like keeping the right amount of oxygen in your cells.
Prolyl hydroxylase domain enzymes (PHD1, 2, and 3) are the primary mammalian sensors for oxygen in the blood and tissues when oxygen levels are normal (normoxia) or low (hypoxia). Oxygen binding to the PHD acts as an "on" switch for the binding of another molecule to PHD, a molecule called 2-oxoglutarate (2-OG). 2-OG can only bind to PHD if O2is already bound.
When there is sufficient oxygen in the blood and tissues, the PHD is bound by O2 and 2-OG, so the HIFs are degraded. But when there is hypoxia– little O2 and 2-OG are bound to the PHD – and there is no destruction of HIFs.
If they aren’t targeted for the proteasome, HIFs are free to do their job. They turn on genes that work to increase oxygen in the blood and tissues, including making more red blood cells, using more iron for heme, stimulating the production of new blood vessels, and triggering cells to use glycolysis instead of the citric acid cycle because glycolysis doesn’t need O2(here’s a review).
When oxygen levels in blood and tissue return to normal, more O2 will be free to bind to the PHDs and the HIFS will then be degraded. Their effects on genes will be turned off as their concentration decreases.
Prolonged exposure to high levels of O2 (or short exposures to very high levels) can cause cell destruction, collapse of lung alveoli, retinal detachment, and seizures. Scuba divers, firemen, and anyone else using oxygen tanks must be aware of the dangers of either running out of oxygen or breathing in too much.
This is where TRPA1 ion channels enter the picture. A 2008 study showed that TRPA1 can be activated by O2. In hyperoxic situations, there is too much oxygen to be bound up by the available PHDs, so some is left to interact with TRPA1 channels in the membranes of the vagus and sensory neurons, as well as in tissue cells. The O2 can open the TRPA1 channel directly and lead to firing of the neuron.
The more O2 there is, there more activation of TRPA1, and this is good. In hyperoxic situations, the body constricts many blood vessels to limit the excess oxygen getting into the tissues. Hyperoxia also ramps up the cells’ protective mechanisms against reactive oxygen species damage.
What we don’t know is which responses to hyperoxia are controlled by the TRPA1 channel activity. A 2011 study goes onto show that the PHDs lose their inhibitory function on TRPA1 in both hyperoxia and hypoxia. This is a similar conundrum to the one we saw last week - where TRPA1 senses cold, but responds to many of the “hot” agonists of TRPV1. Here, the same sensor is stimulating different responses to too much ortoo little oxygen.
It seems that your TRPA1 cold sensing channels are important for tanning in the summer! A 2013 study shows that a process called phototransduction uses G-protein coupled receptors to stimulate TRPA1 in melanocyte and keratinocyte membranes and results in an influx of calcium. This destabilizes the membranes and facilitates the transfer of melanosomes from melanocytes into the surrounding keratinocytes, as we have talked about before.
Phototransduction in general is where light energy is changed (transduced) into another form of energy. The best example is in the retina of the eye, where rods and cones turn visual light into neural signals that are then processed in the brain as images – that’s how you see. In the skin, the energy of the UV light is turned into chemical energy (flow of ions in and out of channels) to stimulate cellular activity.
A second 2013 study from the same group says that retinol (hear the word retina in there? As in the retina of your eye?) is the photoactive chemical that starts the G-protein couple receptor cascade that then results in TRPA1 activation and melanin synthesis in melanocytes.
A new photosensitive protein called optovin has been identified in zebrafish. It mediates TRPA1 activation via a sensitive part of the TRPA1. Optovin allows for optical control of TRPA1-expressing neurons, meaning - optovin absorbs light, generates singlet oxygen radicals and these interact with the the oxygen-sensitive cysteine residues on TRPA1 and activates the receptor. Sound familiar? This is similar to the binding of oxygen that helps the body recognize and respond to hyperoxia.
In an effort to take advantage of this great system, the research field of optogenetics has been born. Let’s say you want to study what happens when a set of neurons fires. Introduce optovin and TRPA1 (or a similar system, like opsin or retinal) into the appropriate neural pathway and then you can fire them at will just by shining a light on them. Imagine, shine a laser pointer on a mouse and instantly he starts to jump, or drool, or tell a joke.
One last exception for the day – one that will lead to some amazing stories for next week. In mammals and many other animals, TRPA1 senses noxious cold (in addition to the amazing things we just talked about), but in some species it acts completely the opposite.
In mosquitoes, TRPA1 senses heat instead of cold. A 2013 study shows that larval A. gambiae (the mosquitoes that carry malaria) rely on TRPA1. If you decrease the amount of TRPA1, the mosquito larvae don’t exhibit thermal locomotive behaviors that would normally keep them in the preferred temperature of water. This might be important for killing mosquito larvae in water; if you can use antagonists to TRPA1 to move them away from their optimum temperature, they’ll die as babies and never become bloodsuckers.
In fruit flies, TRPA1 is also important for circadian (daily) locomotor activity patterns (2013 study). Instead of having a light/dark drive their activity, the temperature fluctuations between day and night can also serve to entrain circadian cycles. There are activity cells in fly brain for morning, daytime, and evening activity levels. TRPA1 isn’t expressed in morning neurons, so it’s activation by heat is what increases activity in day and evening.
Another 2013 study shows that TRPA1 is used as thermoregulatory control of circadian rhythm in drosophila. Loss of TRPA1 altered behaviors and changed the expression of an important circadian rhythm protein called Per in the pacemaker cells.
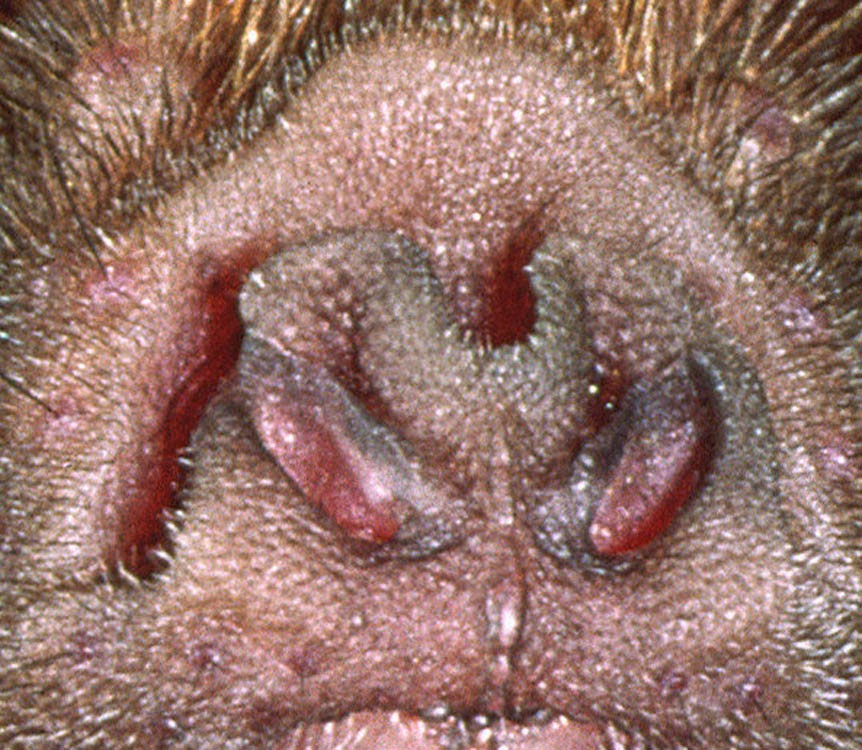
In this one regard, birds are a lot like mosquitoes and flies. Chickens for example, have TRPA1 channels that induce pain due to high heat, just like their TRPV1 channels (which, you remember, don’t react to capsaicin).
A 2014 study showed that chicken TRPA1 is a heat and noxious chemical sensor – it acts opposite to the TRPA1 in humans, even though we are both homeotherms (maintain a body temperature within a small range). Chicken TRPA1 is almost always co-expressed with TRPV1, so they double up on heat but might have less cold sensing – why? - cold can still be damaging to cells so they need to know to avoid it.
The weird TRPA1 of birds lends itself to a bizarre use for us. Methyl anthranilate (MA) is a non-lethal bird repellent, and the same 2014 study shows that it works by activating bird TRPA1 pain sensors. MA doesn’t activate TRPA1 in other species, like humans; three amino acids critical for its MA activity are different in bird and mammal TRPA1.
Since MA doesn’t work on human TRPA1, it can be sprayed on crops to keep birds away – it’s a chemical scarecrow - if it only had a brain! It can also be sprayed on surfaces to keep birds from congregating. MA works as a repellent by stimulating the trigeminal nerves via TRPA1 in the bird’s beak, eyes and throat.
Both MA and diMA are naturally occurring in concord grapes, strawberries, other fruits, and are especially important for flavor of apples. Maybe that’s why they make grapples – grape-flavored apples. Actually, grapples are apples soaked in MA, not a genetic hybrid. DiMA is also released from musk glands of foxes, and is produced in rotting flesh – does spoiling meat taste like apples or grapes to you?
Next week – odd changes in TRPA1 and TRPV1 turn animals into better hunters.
For more information or classroom activities, see:
Oxygen sensing –
Proteasome –
Phototransduction –
Optogenetics –
Methyl anthranilate –